Editorial Policies
1. Open access policy
This journal provides immediate open access to its content on the principle that making research freely available to the public supporting a greater global exchange of knowledge.
Open Access Policy is based on rules of Budapest Open Access Initiative (http://www.budapestopenaccessinitiative.org/. ) Balkan Medical Journal (Trakya University) applies the Creative Commons Attribution (CC BY NC ND) license to articles we publish. If you submit your paper for publication, you agree to have the CC-BY-NC-ND license applied to your work. Under this Open Access license, you as the author agree that anyone can copy, distribute or reuse the content of your article for non-commercial purposes for free as long as the author and original source are properly cited. The corresponding author must sign the Creative Commons License Agreement after their articles are accepted.
Copyright Policy
The Balkan Medical Journal is protected by copyright law. The Copyright Agreement Form should be signed and attached to submissions. As of 2021, the journal is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 (CC BY-NC-ND 4.0) International License. By signing this form, the authors agree that the article, if accepted for publication in the Balkan Medical Journal, will be licensed under a CC BY-NC-ND 4.0 license, which permits third parties to copy, distribute, or reuse the content in unadapted form only for non-commercial purposes, provided the author and original source are properly cited.
Authors hold both the scientific and ethical liability, as well as the copyright for the manuscripts. Commercial rights to the manuscripts are transferred to the Trakya University School of Medicine if the article is accepted for publication.
The CC BY-NC-ND license includes the following elements:
BY – Credit must be given to the creator
NC – Only noncommercial uses of the work are permitted
ND – No derivatives or adaptations of the work are permitted
2. Editorial process and peer review policy
All manuscripts submitted for publication are strictly reviewed for their originality, methodology, importance, quality, ethical nature and suitability for the journal. The Balkan Medical Journal uses a well-constructed scheme for the evaluation process. The editor-in-chief has full authority over the editorial and scientific content of the Balkan Medical Journal and the timing of publication of the content.
The whole editorial and peer review process can be divided into 20 successive steps:
1. 1st technical review by the Editorial Secretary
2. 1st review by the Editor-in-Chief: [immediate reject, immediate revision or further evaluation]
3. 1st review by the Associate Editors
4. 1st similarity check
5. 1st evaluation at the weekly Editorial Board Meeting
6. 2nd review by the Editor-in-Chief: [immediate reject, immediate revision or further evaluation]
7. 2nd detailed technical review by the Editorial Secretary
8. 1st review by two or more external reviewers
9. 1st review by a Biostatistician
10. Revisions (if needed)
11. 2nd similarity recheck
12. 2nd evaluation by the Associate Editors
13. 2nd evaluation at the weekly Editorial Board Meeting
14. 3rd review by the Editor-in-Chief [reject or revision]
15. Assignment of DOI number
16. Copy editing
17. Galley proof preparation
18. Final review by the Editorial Secretary, Associate and Biostatics Editors and the Editor-in-Chief
19. 3rd similarity check
20. Publication
Technical reviews are typically processed in a few days after submission. The initial evaluation usually takes 1 to 3 weeks in 90% of the manuscripts. However, nearly 80% of manuscripts are rejected at this stage. For stage 7, it depends on authors’ attention to the instructions and the time they provide the absent documents. The publication process after submission may take 1 to 12 months. However, all accepted manuscripts are assigned with a DOI number following their acceptance and copy editing, and they are published Ahead of Print prior to their inclusion in their scheduled issue.
Very few manuscripts are rejected at the 2nd stage. About one-fifth of manuscripts are accepted for further evaluation at the 6th stage. The remaining manuscripts may be accepted at the 14th stage.
Plagiarism detection
Plagiarism is a serious problem and the most common ethical issue afflicting medical writing. The Balkan Medical Journal does not allow any form of plagiarism. In accordance with our journal policy, submitted manuscripts are screened with plagiarism software to detect instances of overlapping and similar text (iThenticate and others) at least two times (during the evaluation process and after acceptance). High similarity scores may lead rejection of a manuscript before and even after acceptance. Depending on the type of article and the percentage of similarity score taken from each article, the overall similarity score is generally expected to be less than 20 or 25%.
Clinical trials and reporting guidelines
The Balkan Medical Journal encourages the registration of all clinical trials via ClinicalTrials.gov (www.clinicaltrials.gov) or one of the registries of the WHO’s International Clinical Trials Registry Platform (ICTRP: http://www.who.int/ictrp/network/primary/en/index.html). Especially the phase 3 clinical trials must be registered at or before the time of first patient enrollment. The name of the registry and the registration number together with the information of funding source should be provided at the end of the abstract.
Authors should refer to the guidelines below when preparing their manuscript:
.png)
For further information on the reporting guidelines for health research, authors are suggested to refer to the EQUATOR network website (http://www.equator-network.org/)
3. Ethics
The Editorial Board of the Balkan Medical Journal and the Publisher adheres to the principles of the International Council of Medical Journal Editors (ICMJE), the World Association of Medical Editors (WAME), the Council of Science Editors (CSE), the Committee on Publication Ethics (COPE), the US National Library of Medicine (NLM), the World Medical Association (WMA), the US Office of Research Integrity (ORI), the European Association of Science Editors (EASE), and the International Society of Managing and Technical Editors (ISMTE).
In accordance with the journal's policy, an approval of research protocols by an ethics committee in accordance with international agreements “WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects (last updated: October 2013, Fortaleza, Brazil)” , "Guide for the care and use of laboratory animals (8th edition, 2011)" and/or “International Guiding Principles for Biomedical Research Involving Animals (2012)” is required for all research studies. If the submitted manuscript does not include ethics committee approval, it will be reviewed according to COPE's guideline (Guidance for Editors: Research, Audit and Service Evaluations). If the study should have ethical approval, authors will be asked to provide ethical approval in order to proceed the review process. If they cannot provide ethical approval, their manuscript will be rejected and also their institutions and when needed, the related bodies in their country will be informed that such studies must have ethics committee approval. If they provide approval, review of the manuscript will continue.
If the study does not need ethics committee approval after the editorial board’s review, the authors will be asked to provide an ethics committee approval or a document given by a related independent committee that indicates the study does not need ethics committee approval according to the research integrity rules in their country. If the authors provide either an approval or a document showing that ethics approval is not needed, the review process can be continued. If the authors cannot provide either documents, the manuscript may be rejected.
For articles concerning experimental research on humans, a statement should be included that shows informed consent of patients and volunteers was obtained following a detailed explanation of the procedures that they may undergo. The journal may request a copy of the Ethics Committee Approval received from the relevant authority. Informed consent must also be obtained for case reports and clinical images. More details on the ethical principles of the journal may be found at the "Ethical Guidelines" and the "Instructions to Reviewers" pages.
All authors should meet the ICMJE’s authorship criteria outlined at the “authorship contribution form” section. Balkan Medical Journal does not accept gift, guest, or ghost authorship, and will act according to the COPE guidelines and flowcharts when faced with cases of suspected misconduct.
Conflict of interest
The Balkan Medical Journal’s editorial review process is in accordance with the Good Editorial Practice set by international editorial organizations (ICMJE, EASE, WAME, COPE, CSE,…). WAME indicates that “conflict of interest exists when an author, reviewer, or editor in the publication process (submission of manuscripts, peer review, editorial decisions, and communication between authors, reviewers and editors) has a competing interest that could unduly influence his or her responsibilities (academic honesty, unbiased conduct and reporting of research, and integrity of decisions or judgments) in the publication process”.
The Balkan Medical Journal requires that each author, reviewer, and editor must disclose to the editor-in-chief any conflict of interest related to family, personal, financial, political or religious issues as well as any competing interest outlined above at the WAME’s definition. Whether or not a conflict of interest and financial support exist, they must be declared at the ICMJE Conflict of Interest form as well as at the end of the manuscripts. If a reviewer, an associate editor, or a section editor has a conflict of interest and/or believes that it is not appropriate to be a reviewer, or an editor for a given manuscript, the reviewer or the editor should resign from the assignment. The Editorial Board members of the Balkan Medical Journal, who handle submissions and recommend decisions to the Editor in chief (EIC, Managing Editor and Section Editors) may also submit their own manuscripts to the journal as all of them are active researchers and scientists. However, they cannot take place at any stage on the editorial decision of their manuscripts in order to minimize any possible bias. They will be treated like any other author, and if any, final acceptance of such manuscripts can only be made by the positive recommendation of at least two external reviewers, who are not the members of decision-making Editorial Board.
Authors should not contact any of the section editors during the review process. All necessary information regarding the process of a manuscript can be obtained from the editorial office. However, the names of the handling editor and the reviewers are not given to the author(s). Due to the Balkan Medical Journal’s double-blinded review principles, the names of authors and reviewers are not known to the other.
Please refer to the “conflict of interest statement and copyright form” section below for the conflict of interest declaration for authors. For a conflict of interest statement for reviewers, please refer to the “Instructions to Reviewers” page.
4. Data-sharing policy
Balkan Medical Journal follows ICMJE recommendations. The ICMJE require authors to submit a data-sharing statement for randomized clinical trials. Authors must provide a data-sharing notification to indicate whether data will be shared. According to ICMJE “Data sharing statements must indicate the following: whether individual deidentified participant data (including data dictionaries) will be shared; what data in particular will be shared; whether additional, related documents will be available (e.g., study protocol, statistical analysis plan, etc.); when the data will become available and for how long; by what access criteria data will be shared (including with whom, for what types of analyses, and by what mechanism)” (for details: http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html.
Balkan Medical Journal require authors to submit a data-sharing statement form and register a data-sharing plan when registering a clinical trial on or after Jan 1, 2019.
| Availability of data | Template for data-sharing statement |
| Data openly available in a public repository that issues datasets with DOIs | The data that support the findings of this study are openly available in [repository name e.g “figshare”] at http://doi.org/[doi], reference number [reference number]. |
| Data openly available in a public repository that does not issue DOIs | The data that support the findings of this study are openly available in [repository name] at [URL], reference number [reference number]. |
| Data derived from public domain resources | The data that support the findings of this study are available in [repository name] at [URL/DOI], reference number [reference number]. These data were derived from the following resources available in the public domain: [list resources and URLs] |
| Embargo on data due to commercial restrictions | The data that support the findings will be available in [repository name] at [URL / DOI link] following an embargo from the date of publication to allow for commercialization of research findings. |
| Data available on request due to privacy/ethical restrictions | The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. |
| Data subject to third party restrictions | The data that support the findings of this study are available from [third party]. Restrictions apply to the availability of these data, which were used under license for this study. Data are available [from the authors / at URL] with the permission of [third party]. |
| Data available on request from the authors | The data that support the findings of this study are available from the corresponding author upon reasonable request. |
| Data sharing not applicable – no new data generated | Data sharing is not applicable to this article as no new data were created or analyzed in this study. |
| Author elects to not share data | Research data are not shared. |
| Data available in article supplementary material | The data that supports the findings of this study are available in the supplementary material of this article. |
5. Preprint
Balkan Medical Journal does not consider preprint publications as prior publications. In other words, authors are allowed to present and discuss their findings on a non-commercial preprint server (e.g., arXiv, bioRxiv, chemRxiv, medRxiv ) before submission to a journal.
Authors must provide the journal with the preprint server deposition of their article accompanying its DOI during initial submission. Preprints cannot be used as a reference in any publication in the Balkan Medical Journal unless cited in a personal communication format, i.e., as an in-text reference (using preprint link, DOI, or both).
If the article is published in the Balkan Medical Journal, it is the responsibility of the authors to update the archived preprint and link it to the published version of the article. The preprint must be removed in the case of acceptance to leave only the final version online.
6. Generative AI and AI-Assisted Technologies Policy
About
The Balkan Medical Journal acknowledges the growing role of generative artificial intelligence (AI) and AI-assisted technologies (“AI Tools”) in biomedical research, writing, and publishing. This policy establishes clear guidance for authors, reviewers, and editors to ensure transparency, accountability, and scientific integrity when such tools are used in any part of the research or publication process.
The journal supports responsible and ethical use of AI technologies that enhance scholarly communication, provided they do not compromise authorship, confidentiality, originality, or the rigor of scientific review.
For Authors
Use of AI Tools in Manuscript Preparation
Authors may use AI Tools responsibly for tasks such as improving clarity, grammar, and structure; synthesizing literature; or organizing ideas. However, these tools must not replace human judgment, interpretation, or analysis. Authors remain fully responsible for the accuracy, originality, and integrity of all submitted content.
Authors must:
• Critically review and verify all AI-generated text, data, and references to ensure accuracy, neutrality, and originality.
• Avoid relying on AI Tools for analysis or interpretation without human validation.
• Ensure that any use of AI Tools complies with data privacy, confidentiality, and intellectual property laws.
• Confirm that AI Tools used do not claim ownership of submitted materials or restrict publication rights.
Disclosure Requirements
Use of any generative AI or AI-assisted tools in manuscript preparation must be declared at submission. A brief AI Disclosure Statement should appear in the manuscript, specifying:
• The name and version of the AI Tool used.
• The purpose of its use (e.g., language editing, literature summarization).
• The extent of human oversight.
Basic language editing (grammar and spelling) does not require disclosure. AI use in research design, analysis, or data generation must be detailed in the Methods section.
Authorship
AI Tools cannot be credited as authors or co-authors, nor cited as such. Authorship implies human accountability for the entire content, including conceptualization, data analysis, interpretation, and approval of the final version. Each listed author must fulfill authorship criteria according to ICMJE Recommendations.
Use of AI Tools in Figures and Images
The Balkan Medical Journal does not permit the use of generative AI or AI-assisted tools to create, modify, or enhance scientific figures or images in submitted manuscripts.
Permitted adjustments include only standard, transparent modifications such as brightness, contrast, or color balance corrections that do not obscure or alter original data.
AI-generated or modified images (e.g., synthetic microscopy images, AI-enhanced blots) are not acceptable unless the use of AI forms part of the study’s validated research methodology. In such cases, the authors must:
• Clearly describe the AI methods used (tool name, model version, developer, parameters).
• Provide raw, unaltered data for editorial evaluation upon request.
AI-generated artwork, cover images, or graphical abstracts are not allowed without prior written approval from the editorial office.
For Reviewers
Peer review is a confidential and human-centered process. Reviewers must not upload any part of a submitted manuscript or their review report into generative AI platforms. Doing so may breach confidentiality, data protection, or intellectual property rights.
Generative AI must not be used to assist in the scientific evaluation or critique of manuscripts, as these tasks require human expertise, context, and ethical judgment. Reviewers are personally responsible for the accuracy and integrity of their reports.
The journal may use in-house or licensed AI-assisted systems (e.g., for reviewer matching or plagiarism checks), all of which comply with data privacy and responsible AI principles.
For Editors
Editors must treat all submitted materials and correspondence as confidential and must not upload manuscripts or editorial communications into public AI Tools. Generative AI must not be used to assist in editorial assessment, decision-making, or correspondence related to manuscript evaluation. All editorial decisions must result from human review and reasoning.
If an editor suspects a violation of this AI policy by an author or reviewer, it should be reported to the Editor-in-Chief or publisher for formal investigation.
The Balkan Medical Journal may use internal AI systems that comply with confidentiality and data protection standards to facilitate workflow tasks such as reviewer matching, plagiarism screening, and metadata checks.
Publication Process and Journal Use of AI
The journal may use AI or AI-assisted tools internally to:
• Support plagiarism and image integrity screening.
• Identify suitable reviewers.
• Perform language and format checks during technical review.
• Detect duplicate or unethical submissions.
All such uses occur under strict human oversight and within controlled systems that comply with privacy and data security requirements. AI Tools are never used for scientific decision-making, acceptance judgments, or editorial conclusions.
Final Responsibility
In all cases, human oversight and accountability remain central. The use of AI Tools must not diminish author responsibility, reviewer integrity, or editorial judgment.
Transparency in AI use fosters trust among researchers, readers, and the scientific community, ensuring that the Balkan Medical Journal continues to uphold the highest ethical and professional standards in medical publishing.
7. Sex and gender policy
“The Balkan Medical Journal has recently established a policy that requires all authors to ensure proper representation of gender and/or sex as a variable for all study types.
Authors are strongly recommended to use the terms sex (for reporting biological factors) and gender (for reporting identity, psychosocial, or cultural factors) correctly. Wherever possible, the sex and/or gender of the study participants should be reported and the methods used to determine sex and/or gender should be explained. Regardless of whether the result is favorable or not, routine sex- and gender -separated data should be presented at the appropriate instances. In addition to data limitations, the potential implications of sex and gender on study results/analyses should be discussed. Please see the table for SAGER recommendations and refer to the SAGER guideline for further information.
Sex and Gender Equity in Research (SAGER) guidelines*
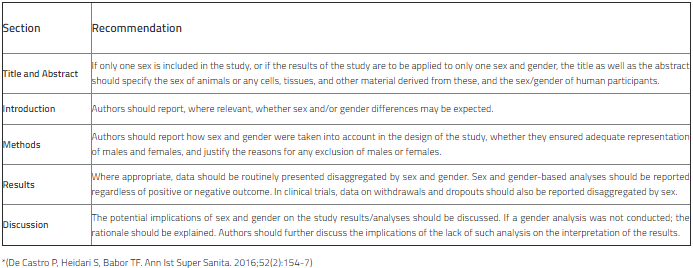
8. Change of authorship and withdrawal request
Change of Authorship
Any request to change the author list after submission, such as a change in the order of the authors or the deletion or addition of author names, is subject to the Editorial Board’s approval. To obtain this approval, please find and complete the change of authorship form on the Journal’s website and send it to the Journal’s office. This form should include the following information:
The reason for the change of authorship
Signatures of all authors (including the new and/or removed author)
Please note, if you are adding or removing author/authors, a new copyright transfer form signed by all authors should also be sent to the editorial office after the Editorial Board approves the change of authorship.
Withdrawal Policy
Balkan Medical Journal is committed to providing high quality articles and uphold the publication ethics to advance the intellectual agenda of science. We expect our authors to comply with, best practice in publication ethics as well as in quality of their articles.
Withdrawal of a manuscript will be permitted only for the most compelling and unavoidable reasons. For withdrawal of a manuscript authors need to submit an "Article withdrawal Form", signed by all authors mentioning the reason for withdrawal to the Editorial Office. The form is available from the web page of the journal. Authors must not assume that their manuscript has been withdrawn until they have received appropriate notification to this effect from the editorial office.
In a case where a manuscript has taken more than six months’ time for review process, that allows the author to withdraw manuscript.
Manuscript withdrawal penalty: After receiving the Article withdrawal Form, Balkan Medical Journal Editorial Board will investigate the reason of withdrawal.
If the reason finds to be acceptable, the author is allowed to withdraw the manuscript without paying any withdrawal penalty. If not Balkan Medical Journal will not accept any manuscripts from the same author for one year.
Important notes: Manuscripts may be withdrawn at any stage of review and publication process by submitting a request to the editorial office. Manuscript withdrawal will be permitted after submission only for the most compelling and unavoidable reasons.
If the author wants to withdraw a manuscript, the author needs to submit a completed "Article withdrawal Form", signed by all authors of the manuscript stating the reasons for manuscript withdrawal. The form is available from the web page of the journal.
The manuscript will not be withdrawn from publication process until a completed, signed form is received by the editorial office. Authors must not assume that their manuscript has been withdrawn until they have received appropriate notification to this effect from the Balkan Medical Journal editorial office.
9. Appeals and complaints
Appeal and complaint cases are handled within the scope of COPE guidelines by the Editorial Board of the journal. Appeals should be based on the scientific content of the manuscript. The final decision on the appeal and complaint is made by Editor in Chief. An Ombudsperson or the Ethical Editor is assigned to resolve cases that cannot be resolved internally. Authors should get in contact with the Editor in Chief regarding their appeals and complaints via e-mail at [email protected].